Ocean acidification is of particular concern in the northern Pacific Ocean.
Contributions from the burning of fossil fuels have pushed levels of carbon dioxide—a greenhouse gas—to change more rapidly over the past 200 years of the Industrial Era than for the 650,000 years preceding that Era.
The effects of carbon dioxide (CO2) on Earth’s climate have been mitigated by the oceans, which absorb roughly 25 percent of the CO2 contributed to the atmosphere by human activities each year. Increasing levels of carbon dioxide, however, are outstripping the absorptive abilities of the oceans and are causing climate change.
A more recently recognized effect is ocean acidification. Although climate change and ocean acidification are linked by the same cause—increasing levels of CO2—they are different phenomena. Ocean acidification occurs when carbon dioxide reacts with seawater and forms carbonic acid. In short, the oceans are becoming more acidic.
In an excellent brochure about ocean acidification, scientist Dick Feely with NOAA’s Pacific Marine Environmental Laboratory in Seattle, and his colleagues, write “…ocean acidification is a straightforward chemical response to CO2 emissions, and is measured and predicted with a high degree of certainty.”
As the oceans have removed CO2 from the atmosphere, the waters at the ocean’s surface have become more acidic. Levels of pH—a measure of acidity, with more acidic at a low pH (2 being lemon juice) and more basic at the high end (12 being oven cleaner)—have decreased. The historical pH of seawater is 8.16. Today’s measurements show a pH of 8.05. Even this seemingly small change can have deleterious effects on marine organisms that use the chemicals in seawater to build shells and skeletons made of the mineral calcium carbonate. Some of these organisms, such as the tiny, one-celled animals called pteropods, are at the base of the marine food chain. Disturbances to pteropod populations have ramifications for the entire ecosystem. Other organisms such as corals and mussels, which construct their structures and shells of calcium carbonate, could be affected. Fish populations may be affected by acidosis, a buildup of carbonic acid in body fluids.
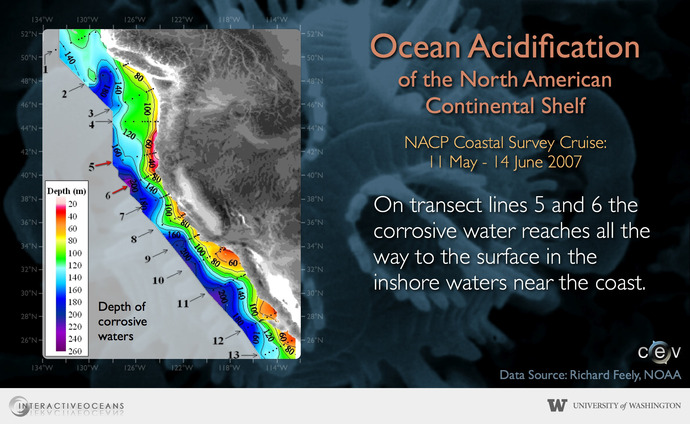
Ocean acidification is of particular concern in the northern Pacific Ocean. Owing to the changes in climate over the past 50 years, this region has transitioned from a primarily cold and icy Arctic ecosystem to today’s warmer, sub-Arctic conditions. Already made vulnerable by climate change, the changing ocean chemistry of this region is expected to affect this region immediately. Because one half of all U.S. shellfish and fish are landed from the North Pacific, a productive and economically significant catch may be at risk because of acidosis.
In looking to the future, Feely and his colleagues write : “Today’s carbon dioxide emissions will continue to affect global ocean chemistry for many hundred of years to come, but a significant effort to curb our emissions will help slow the rate of change, allowing ecosystems a better chance to adapt and decreasing our ultimate negative impact on the environment.”
For more information: The Ocean in a High CO2 World Symposium.